-
Notifications
You must be signed in to change notification settings - Fork 3
Desirable Compounds Not Yet Synthesised
This part of the wiki tracks suggestions of molecules that have yet to be completed. To suggest a molecule, it's best to start an Issue (tab above; use the tag "Molecule Suggestion") so that the suggestion can be explained/justified/debated. If the suggestion is good, the molecule can be added below, the original Issue can be linked and the Issue can be closed.
The Chase Smith benzamide compounds have been moved to here
Example synthetic planning threads: Nov 2015, May 2018
The Tianyi compound (contains methylated imidazopyrazine core, analogous to MMV669846): Discussion of Desirability: GHI358, GHI390 Haverford Planning OC(C1=CC=CC=C1)COC2=CN=CC3=NC(C)=C(C4=CC=C(OC(F)F)C=C4)N32 InChI=1S/C22H19F2N3O3/c1-14-21(16-7-9-17(10-8-16)30-22(23)24)27-19(26-14)11-25-12-20(27)29-13-18(28)15-5-3-2-4-6-15/h2-12,18,22,28H,13H2,1H3 VGNPLLBRBOUQAA-UHFFFAOYSA-N

The Heterooxazole (unexplored amide isostere in side chain): General discussion of the idea, Proposed synthesis, start of the synthesis, ELN FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(C4=CN=C(C5=CC=CC=C5)O4)N32 (free acid) InChI=1S/C21H13F2N5O2/c22-21(23)29-15-8-6-13(7-9-15)19-27-26-18-12-24-10-16(28(18)19)17-11-25-20(30-17)14-4-2-1-3-5-14/h1-12,21H FBTBZFMQUBZDSN-UHFFFAOYSA-N

The Heterooxadiazole (unexplored amide isostere in side chain): General discussion of the idea, Rationale, Proposed synthesis, Synthesis progress report by Haverford College, Relevant ELN, Revised synthetic route(s), Problems with imine formation, Discussion of potentially anomalous NMR spectrum, Second phase introduction and new ELN, Griffith Uni proposed synthesis FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(C4=NC(C5=CC=CC=C5)=NO4)N32 InChI=1S/C20H12F2N6O2/c21-20(22)29-14-8-6-13(7-9-14)18-26-25-16-11-23-10-15(28(16)18)19-24-17(27-30-19)12-4-2-1-3-5-12/h1-11,20H HFPKPTYSFARKAA-UHFFFAOYSA-N

The Alkyl (Test to see if heteroatoms are needed on left-hand-side). Length may need tweaking - MMV669304 is potent): 1st proposed synthesis with commencement and ELN, 2nd proposed synthesis with ELN, 3rd proposed synthesis (Griffith Uni) FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CCC4=CC=CC=C4)N32 InChI=1S/C20H16F2N4O/c21-20(22)27-17-10-7-15(8-11-17)19-25-24-18-13-23-12-16(26(18)19)9-6-14-4-2-1-3-5-14/h1-5,7-8,10-13,20H,6,9H2 QKTHSIJWBSHMDZ-UHFFFAOYSA-N

The Internal Alkyne (related to alkyl): Opening synthesis, ELN, discussion of rationale, 2nd proposed synthesis (Griffith Uni) FC(F)OC1=CC=C(C2=NN=C3C=NC=C(C#CC4=CC=CC=C4)N32)C=C1 InChI=1S/C20H12F2N4O/c21-20(22)27-17-10-7-15(8-11-17)19-25-24-18-13-23-12-16(26(18)19)9-6-14-4-2-1-3-5-14/h1-5,7-8,10-13,20H KNVACGGMAGPRRW-UHFFFAOYSA-N

The Internal Alkene/Reduced Alkyl (synthetically related to alkyne and alkyl compounds; discussion of rationale of all three; The SMILES string is only for the cis isomer: Discussed, starting synthesis, Discussion of NMR spectrum of intermediate, ELN, 2nd proposed synthesis (Griffith Uni) FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(/C=C\C4=CC=CC=C4)N32 InChI=1S/C20H14F2N4O/c21-20(22)27-17-10-7-15(8-11-17)19-25-24-18-13-23-12-16(26(18)19)9-6-14-4-2-1-3-5-14/h1-13,20H/b9-6- SGPCUHISMIPDDU-TWGQIWQCSA-N

The 6 (probe of tolerance to ring substitution): Synthesis proposal, Start of synthesis, ELN. Second phase Haverford attempt with second ELN FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC(C)=C(OCC(O)C4=CC=CC=C4)N32 InChI=1S/C21H18F2N4O3/c1-13-20(29-12-17(28)14-5-3-2-4-6-14)27-18(11-24-13)25-26-19(27)15-7-9-16(10-8-15)30-21(22)23/h2-11,17,21,28H,12H2,1H3 ZYFKQWHTACKGJR-UHFFFAOYSA-N

The 8 (also a probe of tolerance to ring substitution): Haverford synthesis plan, Start of Haverford synthesis, ELN, Rationale, Summary and closure of first attempt, cross-link to new attempt at 6- and 8- FC(F)OC(C=C1)=CC=C1C2=NN=C3C(C)=NC=C(OCC(O)C4=CC=CC=C4)N32 InChI=1S/C21H18F2N4O3/c1-13-19-25-26-20(15-7-9-16(10-8-15)30-21(22)23)27(19)18(11-24-13)29-12-17(28)14-5-3-2-4-6-14/h2-11,17,21,28H,12H2,1H3 ASWYMQOLUDKKDJ-UHFFFAOYSA-N

The Burns (amide isostere): Proposed synthesis from Haverford College, Start of synthesis, Discussion of results of intermediate steps here and here, ELN including summary of outcomes of first attempt. Start of Second Attempt with new ELN. FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(C(C(F)(F)F)NCC4=CC=CC=C4)N32 InChI=1S/C21H16F5N5O/c22-20(23)32-15-8-6-14(7-9-15)19-30-29-17-12-27-11-16(31(17)19)18(21(24,25)26)28-10-13-4-2-1-3-5-13/h1-9,11-12,18,20,28H,10H2 NYRGMZUFRGWPBF-UHFFFAOYSA-N

The Ketone (analog of amide linker): Haverford College proposed syntheses, Start of synthesis, ELN. Also "ketone linker" folder in Ed Tse's lab notebook. Second Haverford College campaign with new ELN. FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(C(CC4=CC=CC=C4)=O)N32 InChI=1S/C20H14F2N4O2/c21-20(22)28-15-8-6-14(7-9-15)19-25-24-18-12-23-11-16(26(18)19)17(27)10-13-4-2-1-3-5-13/h1-9,11-12,20H,10H2 KALFSUSYOZTBSP-UHFFFAOYSA-N

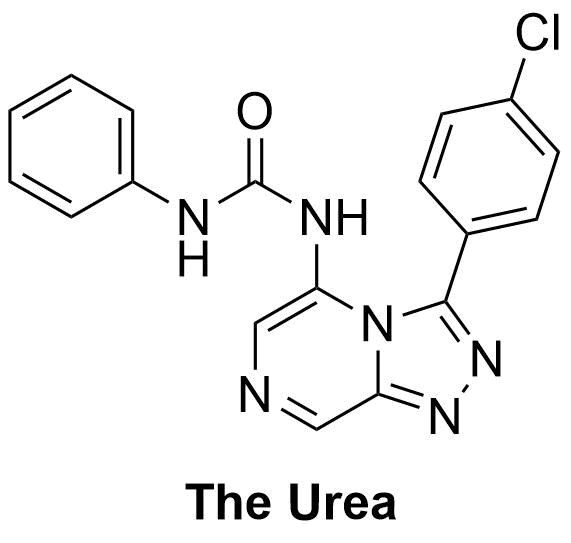
The Urea was suggested here. Further planning here ClC(C=C1)=CC=C1C2=NN=C3C=NC=C(NC(NC4=CC=CC=C4)=O)N32 InChI=1S/C18H13ClN6O/c19-13-8-6-12(7-9-13)17-24-23-16-11-20-10-15(25(16)17)22-18(26)21-14-4-2-1-3-5-14/h1-11H,(H2,21,22,26) MNPRTDCYYNHGPG-UHFFFAOYSA-N
The Carbamate was suggested here and planning here [H]N(C(=O)OC1=CN=CC2=NN=C(N12)C1=CC=C(Cl)C=C1)C1=CC=CC=C1 InChI=1S/C18H12ClN5O2/c19-13-8-6-12(7-9-13)17-23-22-15-10-20-11-16(24(15)17)26-18(25)21-14-4-2-1-3-5-14/h1-11H,(H,21,25) InChIKey=XHDAJNDREKWQAL-UHFFFAOYSA-N

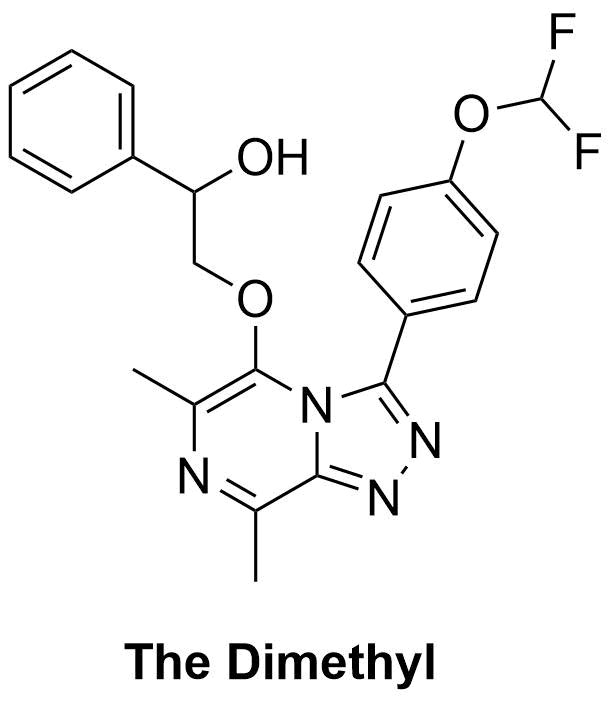
The Dimethyl proposed synthesis from Haverford College. FC(F)OC(C=C1)=CC=C1C2=NN=C3C(C)=NC(C)=C(OCC(O)C4=CC=CC=C4)N32 InChI=1S/C22H20F2N4O3/c1-13-19-26-27-20(16-8-10-17(11-9-16)31-22(23)24)28(19)21(14(2)25-13)30-12-18(29)15-6-4-3-5-7-15/h3-11,18,22,29H,12H2,1-2H3 SPPBNNNUJINLLF-UHFFFAOYSA-N
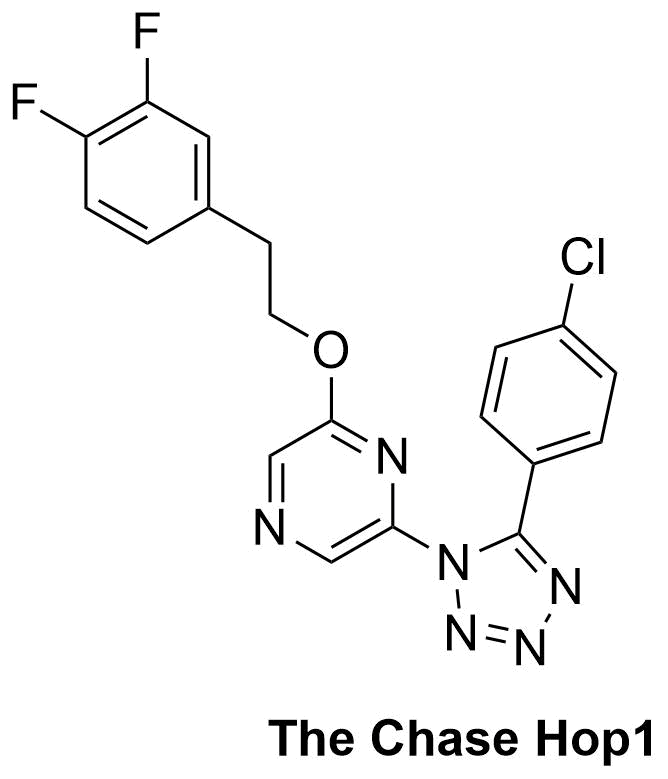
The Chase Hop1 was suggested here. ClC(C=C1)=CC=C1C2=NN=NN2C3=CN=CC(OCCC4=CC(F)=C(F)C=C4)=N3 InChI=1S/C19H13ClF2N6O/c20-14-4-2-13(3-5-14)19-25-26-27-28(19)17-10-23-11-18(24-17)29-8-7-12-1-6-15(21)16(22)9-12/h1-6,9-11H,7-8H2 HHZUPBCYYAXEQO-UHFFFAOYSA-N
The Reading Uni Northeasts by way of Buchwald-Hartwig aminations. Initial Progress Oct 2016, progress update from Sept 2017, new campaign in Oct 2017.

Aims, Concerns and Current Interest in Series 4
Modification of Core Triazolopyrazine
Modification of Pyrazine Substitution Pattern
Modification of the Triazole Substitution
Pyrazine Side Chain Modifications - Ethers
Pyrazine Side Chain Modifications - Amides
Pyrazine Side Chain Modifications - Reversed Amides
Pyrazine Side Chain Modifications - Others
Biological Data Currently not Incorporated into the Main Wiki Sections
Mechanism of Action: Possible PfATP4 Activity Deduced from Parasite Ion Regulation Assays
Synthesis of the Ether-Linked Series
Synthesis of the Amide-Linked Series
Synthesis of the Reverse Amide- Linked Series
Synthesis of Benzylic Functionalised Ether-Linked Series
Alternative Routes to the Triazolopyrazine Core
Triazolopyrazine telesubstitution
Chirality/Stereogenic Centres in This Series
Other Sources of Compounds Relevant to this Series
Desirable Compounds Not Yet Synthesised