You signed in with another tab or window. Reload to refresh your session.You signed out in another tab or window. Reload to refresh your session.You switched accounts on another tab or window. Reload to refresh your session.Dismiss alert
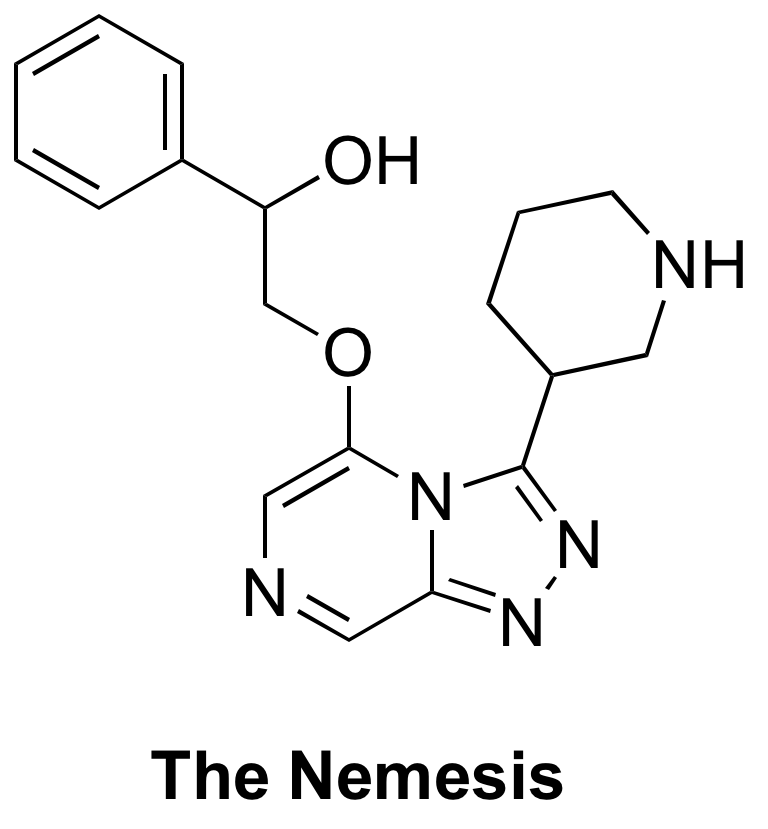
The Nemesis is a key compound for the series originally designed to investigate deplanarisation of the RHS group. The synthesis was picked up by Haverford College (#409) with the THP protected alcohol coupling attempted but purification was not attempted due to time constraints (#430). The synthesis was picked up again in the next year with the deprotection successful by NMR/MS (#466). Unfortunately, not enough of the product was isolated for further analysis/biological evaluation.
I have followed the same route outlined in the above attempts, with the exception of the final deprotection step. Instead of the standard THP deprotection conditions (HCl in dioxane), I used standard Boc deprotection conditions (TFA in DCM). I have also made the di-fluorophenyl derivative of the Nemesis outlined below as a comparison.
The Nemesis
OC(C1=CC=CC=C1)COC2=CN=CC3=NN=C(C4CNCCC4)N32
InChI=1S/C18H21N5O2/c24-15(13-5-2-1-3-6-13)12-25-17-11-20-10-16-21-22-18(23(16)17)14-7-4-8-19-9-14/h1-3,5-6,10-11,14-15,19,24H,4,7-9,12H2
SNDLBGDEOQPBOQ-UHFFFAOYSA-N
The di-fluorophenyl derivative
FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(C4CNCCC4)N32)=C1
InChI=1S/C18H19F2N5O/c19-14-4-3-12(8-15(14)20)5-7-26-17-11-22-10-16-23-24-18(25(16)17)13-2-1-6-21-9-13/h3-4,8,10-11,13,21H,1-2,5-7,9H2
DRTAQDBSMNPECX-UHFFFAOYSA-N
The text was updated successfully, but these errors were encountered:
The Nemesis is a key compound for the series originally designed to investigate deplanarisation of the RHS group. The synthesis was picked up by Haverford College (#409) with the THP protected alcohol coupling attempted but purification was not attempted due to time constraints (#430). The synthesis was picked up again in the next year with the deprotection successful by NMR/MS (#466). Unfortunately, not enough of the product was isolated for further analysis/biological evaluation.
I have followed the same route outlined in the above attempts, with the exception of the final deprotection step. Instead of the standard THP deprotection conditions (HCl in dioxane), I used standard Boc deprotection conditions (TFA in DCM). I have also made the di-fluorophenyl derivative of the Nemesis outlined below as a comparison.
ELN entries for the condensation reaction, core cyclisation, SN2 displacement (Nemesis/difluoro derivative) and deprotection (Nemesis/difluoro derivative).
ELN entries for the alcohol fragments can be found by following the links in the respective SN2 displacement pages.
The Nemesis 1H,13C,COSY,HSQC,HMBC,LRMS and HRMS (pdfs attached)
The di-fluorophenyl derivative 1H,13C,19F,COSY,HSQC,HMBC,LRMS and HRMS (pdfs attached)
The two compounds will be sent off for biological evaluation in the next batch.
Removed from the DCNYS section of the wiki
The Nemesis (deplanarisation of right-hand-side): Haverford College synthetic plan, Start of first Haverford synthesis, ELN, Second phase of synthesis with same ELN.
OC(C1=CC=CC=C1)C OC2=CN=CC3=NN=C(C4CNCCC4)N32
InChI=1S/C18H21N5O2/c24-15(13-5-2-1-3-6-13)12-25-17-11-20-10-16-21-22-18(23(16)17)14-7-4-8-19-9-14/h1-3,5-6,10-11,14-15,19,24H,4,7-9,12H2
SNDLBGDEOQPBOQ-UHFFFAOYSA-N
----------------------------------------------------------------------------------------------------Strings
The Nemesis
OC(C1=CC=CC=C1)COC2=CN=CC3=NN=C(C4CNCCC4)N32
InChI=1S/C18H21N5O2/c24-15(13-5-2-1-3-6-13)12-25-17-11-20-10-16-21-22-18(23(16)17)14-7-4-8-19-9-14/h1-3,5-6,10-11,14-15,19,24H,4,7-9,12H2
SNDLBGDEOQPBOQ-UHFFFAOYSA-N
The di-fluorophenyl derivative
FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(C4CNCCC4)N32)=C1
InChI=1S/C18H19F2N5O/c19-14-4-3-12(8-15(14)20)5-7-26-17-11-22-10-16-23-24-18(25(16)17)13-2-1-6-21-9-13/h3-4,8,10-11,13,21H,1-2,5-7,9H2
DRTAQDBSMNPECX-UHFFFAOYSA-N
The text was updated successfully, but these errors were encountered: